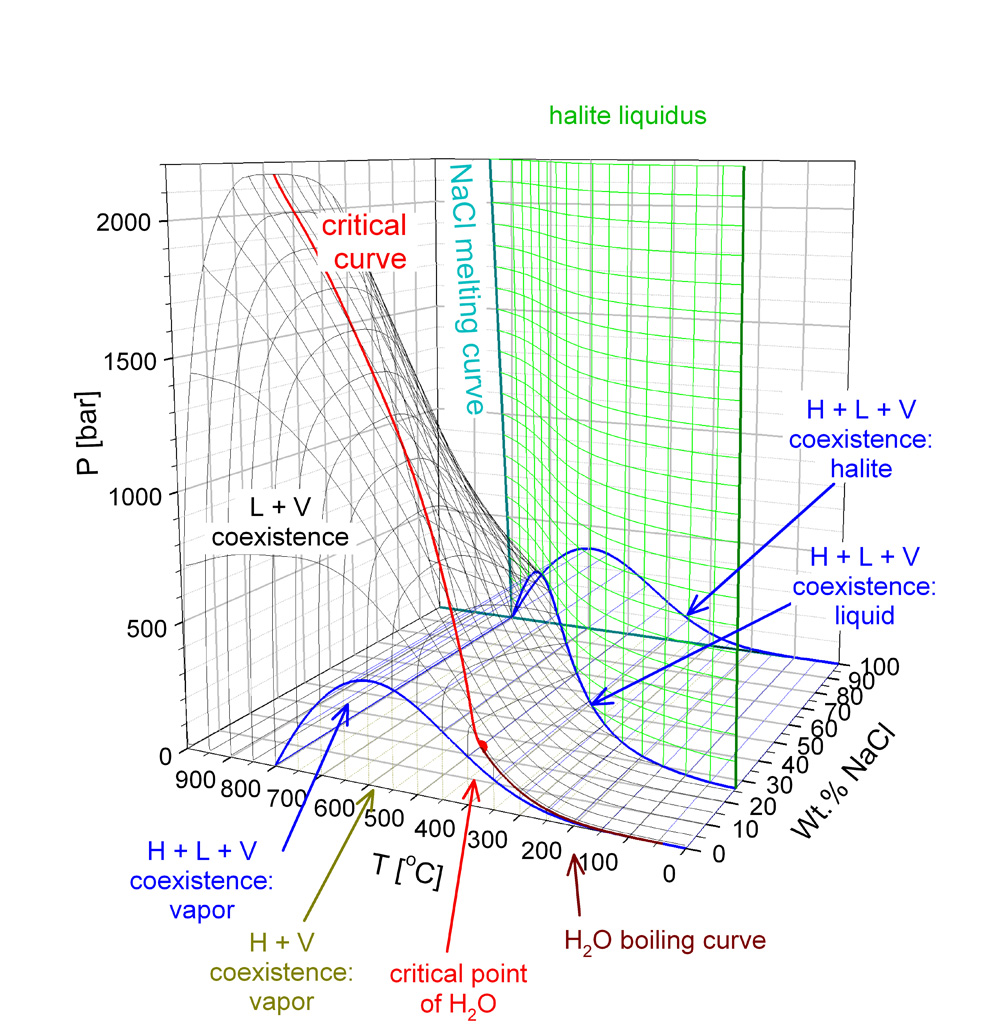
H2O-NaCl fluid properties
Fluids, mainly water with dissolved salts, play an outstanding role in the dissolution, transport, and localized deposition of ore metals. It is therefore of prime interest for our group to be able to model the transport and chemical properties of such fluids over the whole range of pressure (P) - temperature (T) - composition (X) conditions under which hydrothermal ore deposits form.Traditionally, such modelling has been carried out with mathematical models (so-called equations of state) for pure water. However, the binary system NaCl-H2O is a simple yet much more realistic proxy to most fluids involved in hydrothermal, ore-forming processes. Characteristic features that make this system substantially different from pure water are:
- The liquid+vapor coexistence field covers a much broader range of P-T conditions and these conditions are frequently encountered in hydrothermal processes in various levels of the Earth's crust.
- The NaCl component provides a ligand, Cl-, that can complex many ore metals and therefore drastically enhance their solubility compared to "pure" water scenarios.
- Depending on the PTX conditions, coexisting vapour and brine phases can be very similar or very different in density, composition, viscosity etc., making the hydrology of multi-phase flow of such fluids in geologicl systems a very complex problem.
- Ligands such as reduced sulphur species maybe enriched in the vapour phase, leading to metal transport in an "unconvential" medium.
For many years, no equation of state or other mathematical model was available that covered the whole range of PTX conditions of interest to us (0 to 800 Celsius, 0 to 500 MPa, 0 to 1 XNaCl) and was accurate and physically realistic at the same time. Therefore, we have developed our own set of equations that provide this functionality.
Publications
The model ("SoWat" for Sodium Chloride-Water) has been published in Geochimica et Cosmochimica Acta.
Driesner, T., and Heinrich, C.A. (2007): The System H2O-NaCl. I. Correlations for molar volume, enthalpy, and isobaric heat capacity from 0 to 1000 degrees C, 1 to 5000 bar, and 0 to 1 X-NaCl. Geochimica et Cosmochimica Acta 71, 4902-4919.
Driesner, T. (2007): The System H2O-NaCl. II. Correlation Formulae for Phase Relations in Temperature-Pressure-Composition Space from 0 to 1000°C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochimica et Cosmochimica Acta 71, 4880-4901.
Several small computer programs related to these papers can be found on the SoWat software page.
Contact
Inst. für Geochemie und Petrologie
Clausiusstrasse 25
8092
Zürich
Switzerland